Digital Pathology Market to Grow at a Robust Pace During 2022-2028
Stratview Research has published a new report titled “Digital Pathology Market” which is segmented By Product (Software, Device [Scanners, Slide Management System], Storage, System), By Application (Drug Discovery & Development, Academic research, Disease Diagnosis, Cancer Cell Detection, Others), By End Use (Hospitals, Biotech & pharma companies, Diagnostic Labs, Academic & research institutes) and Region (North America, Europe, Asia-Pacific, and Rest of the World).
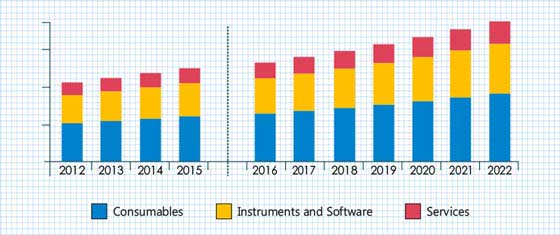
As per the study, the market is expected to grow from USD 831.45 million in 2021 to USD 1816.21 million by 2028 at a CAGR of over 11.8% during the forecast period of 2022-2028.
Read more:
https://www.stratviewresearch.com/1566/digital-pathology-market.html
Digital pathology is referred to as an image-based information setting that is enabled by computer technology which allows the management of information generated via a digital slide. Digitalization of pathology has further led to the automation of tests during disease diagnosis among other applications.
A single digital device can perform tests that were previously carried out using five instruments by pathologists. These solutions offer analysis & scrutiny of images on a computer, online storage of digital slides, and high-resolution sample scanning, which helps pathologists to cross-examine slides without physical evidence.
Report Highlights
This report covers comprehensive data on emerging trends, market drivers, growth opportunities, and restraints that can affect the market dynamics of the industry. It provides a thorough analysis of the market segments which include products, applications, and competitor analysis.
The market is bifurcated into various segments. Below given is the segment-wise analysis -
Product Trends
The Device Segment witnessed the highest market share of more than 50% in 2021 and is projected to register a healthy CAGR during the assessment period.
The market is segmented into software, device, storage, and system. This is mainly ascribed to the rise in academic research activities and improved resolution of slide management systems and scanners, thereby bolstering segment growth.
Digital Pathology is estimated to witness lucrative growth over the forecast period because of the improvements in magnification and slide scanning at the Z-axis, which is offered by whole slide imaging.
In addition, its usage rates are expected to be increased as a result of intuitive user interfaces and high bandwidth connectivity. Major players are constantly introducing technological developments for digital pathology scanners.
Application Trends
The Academic Research Application Segment accounted for the largest market share of more than 45.0% in 2021 and is projected to expand further at a steady CAGR from 2022 to 2028.
The market is segmented into Drug Discovery & Development, Academic research, Disease Diagnosis, Cancer Cell Detection, and Others.
Digital pathology is gaining popularity in various research studies, which is driving up the segment's share. In the future, several cancer therapies are estimated to be introduced, which will further boost the academic research segment. Hospitals, diagnostic labs, biotech and pharmaceutical companies, and academic and research institutes are the main sectors of this market.
End-Use Trends
The Hospital's Segment accounted for the largest revenue share of more than 35% in 2021 owing to the high adoption of digital scanning techniques in hospitals for faster diagnosis and better patient compliance.
The market is segmented into hospitals, diagnostic labs, biotech, and pharma companies, and academic and research institutes based on end-use. In August 2020, Mackenzie Health and Philips teamed up to create one of Canada's first digital pathology platforms to assist patients in receiving faster and more effective care.
Philips' IntelliSite pathology platform will help improve laboratory efficiency, increase access to specialists, and speed up diagnoses.
Collaborations are expected to fuel segment growth in the near future. On the other hand, the diagnostic lab segment is anticipated to experience the fastest CAGR from 2022 to 2028. The biotechnology & pharmaceutical companies segment is anticipated to have steady demand over the period.
According to the study, digital pathology will be used more frequently in drug development, oncology clinical trials, and preclinical GLP pathology over the next decade as a result of cancer and the demand for improved treatment options. Cancer is a significant factor in driving up demand for this segment.
Which Region is expected to remain the largest market?
The North American market accounted for the largest market share of over 40% in 2021 and is likely to grow at a robust CAGR during the review period.
This can be attributed to supportive government initiatives about the development of technologically advanced systems, continual deployment of R&D investments, growing adoption of digital imaging, and the presence of major players boosting the industry growth in the region.
The regional market is expected to grow at a healthy CAGR during the forecast period, thanks to the growing adoption of digital pathology for disease diagnosis and improved reimbursement policies in the U.S. However, Asia Pacific is expected to grow steadily.
The increase in growth can be attributed to the rising penetration of digital imaging in emerging economies, the medical field's investments, and the availability of untapped markets in the Asia Pacific. The healthcare sector in the Asia Pacific has been highly influential due to the increased rate of cancer, which impacts a large population.
Who are the Key Players in the Market?
This report provides market intelligence in the most comprehensive way. It also provides critical insights into the key players active in the market, which will enable strategic decision-making for the existing market players as well as those willing to enter the market. The following are the key players acting in the Digital Pathology Market:
• Danaher (The U.S.)
• Hamamatsu Photonics, Inc (Japan)
• Koninklijke Philips N.V. (Netherlands)
• Olympus Corporation (Japan)
• F. Hoffmann-La Roche Ltd (Switzerland)
• Mikroscan Technologies (The U.S.)
• Inspirata, Inc (The U.S.)
• Visiopharm A/S (Denmark)
• Huron Digital Pathology (Canada)
• 3DHISTECH Ltd. (Hungary)
Key questions answered by the report:
• What is the projected CAGR for revenue from the Digital Pathology Market during the forecast period?
• What would the market be valued at by 2028?
• What is a major driving factor for the growth of the market?
• Which region accounted for the largest revenue share in the Digital Pathology Market?
• Which are the major market players?
Custom Research:
Stratview Research delivers custom research services across sectors. In case of any custom research requirements, please send your inquiry to sales@stratviewresearch.com. Or connect with our experts at +1-313-307-4176.
About Us
Stratview Research is a global market research firm, offering syndicated and custom research reports along with growth consulting services. Our business intelligence and industry research reports offer clients with insightful market data to aid strategic decision-making. These exclusive reports are the result of exclusive research methodology and are available for key industries such as chemicals, composites, advanced materials, technology, renewable energy, and more.
Digital Pathology Market to Grow at a Robust Pace During 2022-2028
Stratview Research has published a new report titled “Digital Pathology Market” which is segmented By Product (Software, Device [Scanners, Slide Management System], Storage, System), By Application (Drug Discovery & Development, Academic research, Disease Diagnosis, Cancer Cell Detection, Others), By End Use (Hospitals, Biotech & pharma companies, Diagnostic Labs, Academic & research institutes) and Region (North America, Europe, Asia-Pacific, and Rest of the World).
As per the study, the market is expected to grow from USD 831.45 million in 2021 to USD 1816.21 million by 2028 at a CAGR of over 11.8% during the forecast period of 2022-2028.
Read more: https://www.stratviewresearch.com/1566/digital-pathology-market.html
Digital pathology is referred to as an image-based information setting that is enabled by computer technology which allows the management of information generated via a digital slide. Digitalization of pathology has further led to the automation of tests during disease diagnosis among other applications.
A single digital device can perform tests that were previously carried out using five instruments by pathologists. These solutions offer analysis & scrutiny of images on a computer, online storage of digital slides, and high-resolution sample scanning, which helps pathologists to cross-examine slides without physical evidence.
Report Highlights
This report covers comprehensive data on emerging trends, market drivers, growth opportunities, and restraints that can affect the market dynamics of the industry. It provides a thorough analysis of the market segments which include products, applications, and competitor analysis.
The market is bifurcated into various segments. Below given is the segment-wise analysis -
Product Trends
The Device Segment witnessed the highest market share of more than 50% in 2021 and is projected to register a healthy CAGR during the assessment period.
The market is segmented into software, device, storage, and system. This is mainly ascribed to the rise in academic research activities and improved resolution of slide management systems and scanners, thereby bolstering segment growth.
Digital Pathology is estimated to witness lucrative growth over the forecast period because of the improvements in magnification and slide scanning at the Z-axis, which is offered by whole slide imaging.
In addition, its usage rates are expected to be increased as a result of intuitive user interfaces and high bandwidth connectivity. Major players are constantly introducing technological developments for digital pathology scanners.
Application Trends
The Academic Research Application Segment accounted for the largest market share of more than 45.0% in 2021 and is projected to expand further at a steady CAGR from 2022 to 2028.
The market is segmented into Drug Discovery & Development, Academic research, Disease Diagnosis, Cancer Cell Detection, and Others.
Digital pathology is gaining popularity in various research studies, which is driving up the segment's share. In the future, several cancer therapies are estimated to be introduced, which will further boost the academic research segment. Hospitals, diagnostic labs, biotech and pharmaceutical companies, and academic and research institutes are the main sectors of this market.
End-Use Trends
The Hospital's Segment accounted for the largest revenue share of more than 35% in 2021 owing to the high adoption of digital scanning techniques in hospitals for faster diagnosis and better patient compliance.
The market is segmented into hospitals, diagnostic labs, biotech, and pharma companies, and academic and research institutes based on end-use. In August 2020, Mackenzie Health and Philips teamed up to create one of Canada's first digital pathology platforms to assist patients in receiving faster and more effective care.
Philips' IntelliSite pathology platform will help improve laboratory efficiency, increase access to specialists, and speed up diagnoses.
Collaborations are expected to fuel segment growth in the near future. On the other hand, the diagnostic lab segment is anticipated to experience the fastest CAGR from 2022 to 2028. The biotechnology & pharmaceutical companies segment is anticipated to have steady demand over the period.
According to the study, digital pathology will be used more frequently in drug development, oncology clinical trials, and preclinical GLP pathology over the next decade as a result of cancer and the demand for improved treatment options. Cancer is a significant factor in driving up demand for this segment.
Which Region is expected to remain the largest market?
The North American market accounted for the largest market share of over 40% in 2021 and is likely to grow at a robust CAGR during the review period.
This can be attributed to supportive government initiatives about the development of technologically advanced systems, continual deployment of R&D investments, growing adoption of digital imaging, and the presence of major players boosting the industry growth in the region.
The regional market is expected to grow at a healthy CAGR during the forecast period, thanks to the growing adoption of digital pathology for disease diagnosis and improved reimbursement policies in the U.S. However, Asia Pacific is expected to grow steadily.
The increase in growth can be attributed to the rising penetration of digital imaging in emerging economies, the medical field's investments, and the availability of untapped markets in the Asia Pacific. The healthcare sector in the Asia Pacific has been highly influential due to the increased rate of cancer, which impacts a large population.
Who are the Key Players in the Market?
This report provides market intelligence in the most comprehensive way. It also provides critical insights into the key players active in the market, which will enable strategic decision-making for the existing market players as well as those willing to enter the market. The following are the key players acting in the Digital Pathology Market:
• Danaher (The U.S.)
• Hamamatsu Photonics, Inc (Japan)
• Koninklijke Philips N.V. (Netherlands)
• Olympus Corporation (Japan)
• F. Hoffmann-La Roche Ltd (Switzerland)
• Mikroscan Technologies (The U.S.)
• Inspirata, Inc (The U.S.)
• Visiopharm A/S (Denmark)
• Huron Digital Pathology (Canada)
• 3DHISTECH Ltd. (Hungary)
Key questions answered by the report:
• What is the projected CAGR for revenue from the Digital Pathology Market during the forecast period?
• What would the market be valued at by 2028?
• What is a major driving factor for the growth of the market?
• Which region accounted for the largest revenue share in the Digital Pathology Market?
• Which are the major market players?
Custom Research:
Stratview Research delivers custom research services across sectors. In case of any custom research requirements, please send your inquiry to sales@stratviewresearch.com. Or connect with our experts at +1-313-307-4176.
About Us
Stratview Research is a global market research firm, offering syndicated and custom research reports along with growth consulting services. Our business intelligence and industry research reports offer clients with insightful market data to aid strategic decision-making. These exclusive reports are the result of exclusive research methodology and are available for key industries such as chemicals, composites, advanced materials, technology, renewable energy, and more.