Absorbable Antibacterial Envelope Market Size, Share, Trends & Forecast to (2024-2032) | UnivDatos
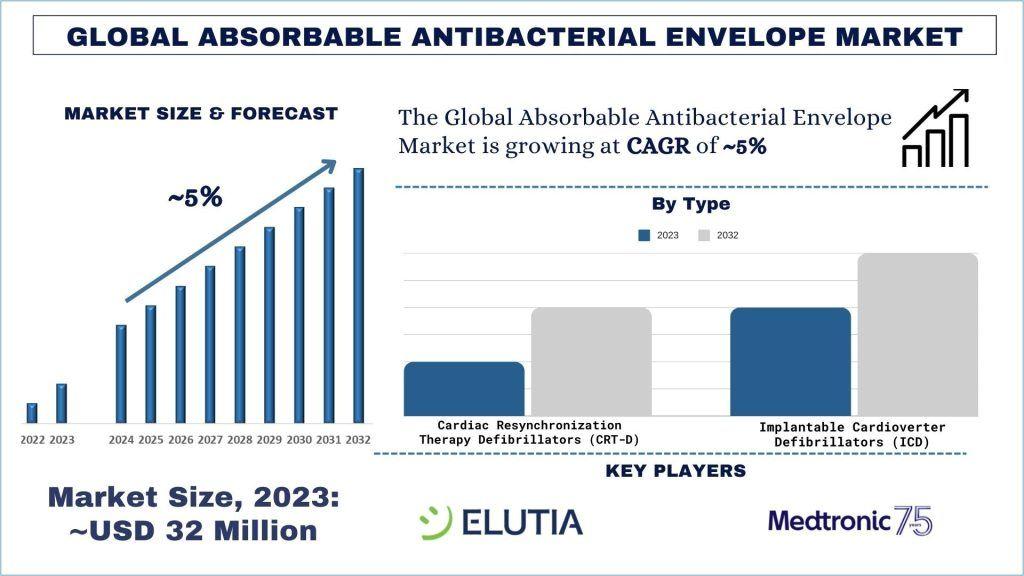
According to the UnivDatos, the increasing elderly population, who are more susceptible to cardiovascular conditions, leads to higher implantation rates of CIEDs, thereby increasing demand for absorbable antibacterial envelopes. will drive the scenario of the global absorbable antibacterial envelope as per their “Global Absorbable Antibacterial Envelope Market” report, the market was valued at USD ~32 million in 2023, growing at a CAGR of ~5% during the forecast period from 2024 - 2032 to reach USD million by 2032.
The U.S. market for absorbable antibacterial envelopes is witnessing significant growth, driven by an increasing prevalence of cardiovascular diseases and a strong emphasis on infection prevention in medical procedures. Absorbable antibacterial envelopes are designed to encase cardiac implantable electronic devices (CIEDs) such as pacemakers and defibrillators, releasing antibiotics to prevent infections.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/absorbable-antibacterial-envelope-market?popup=report-enquiry
Number of individuals with chronic medical conditions in the U.S. from 1995 to 2020 and projections for 2025 and 2050, (Data in Millions):
Demand Drivers
The demand for absorbable antibacterial envelopes in the U.S. is primarily fueled by the high incidence of cardiovascular diseases. According to the American Heart Association, cardiovascular disease remains the leading cause of death in the United States, accounting for approximately 868,000 deaths annually. This high prevalence necessitates the implantation of a significant number of CIEDs, driving the need for effective infection prevention measures.
Moreover, the aging population in the U.S. is contributing to the increased demand. The U.S. Census Bureau projects that by 2030, all baby boomers will be older than age 65, expanding the size of the older population so that one in every five residents will be of retirement age. Older adults are more likely to suffer from cardiovascular diseases and require CIEDs, thereby increasing the market for absorbable antibacterial envelopes.
Technological Innovations and Application
Specifically in the United States, absorbable antibacterial envelopes are mainly used in CIEDs, pacemaker implants, and defibrillators. For example, such devices are essential to people, who have arrhythmias, and heart failure. The antibacterial envelopes surround the CIEDs, which constantly discharge antibiotics to minimize bacterial colonization and subsequent infection of the site that has been implanted with the CIEDs.
However, over the years, with developments in material science and drug delivery technologies, the capability of these envelopes has also improved in terms of their efficacy as well as safety profiles. New procedures are being explored in an attempt to enhance the bio-compatibility of the utilized materials along with the enhanced rates of their absorption while the release of antibiotics is also designed in a manner that provides prolonged antimicrobial protection.
Government Regulations and Standards
It should be noted that the absorption of antibacterial envelopes is considered in the United States of America as a medical product and its circulation is regulated by the Food and Drug Administration (FDA). The Food and Drug Administration which has divided its Center for Devices and Radiological Health into two is in charge of the safety and effectiveness of the absorbable antibacterial envelopes.
Regulatory Pathways:
Normally, absorbable antibacterial envelopes fall under the FDA’s PMA, which is complex and demanding, and proof of efficacy and safety must be offered in support of the product’s requirement. This entails clinical tests and detailed documentation such that the company provides enough proof of how the product conforms to those standards on the market.
Post-Market Surveillance:
After approval regulation, these devices can be set under post-market surveillance to check their efficiency and any negative impacts they have. Manufacturers must also provide the FDA with any information related to the device or events that have occurred and also conduct yearly checks for compliance with the regulations.
Quality Systems Regulation (QSR):
The FDA has a Quality System Regulation that Manufacturers need to follow because it provides guidelines on the design, production, as well as distribution of Medical Devices. Since absorbable antibacterial envelopes are meant to be used to envelope patient’s wounds, this regulation ensures that they are manufactured to the right standards.
Click here to view the Report Description & TOC: https://univdatos.com/reports/absorbable-antibacterial-envelope-market
Conclusion
The demand for absorbable antibacterial envelopes is expected to experience tremendous growth in the U. S. market due to high instances of cardiovascular diseases, a growing population among the elderly, and an advanced healthcare sector. Despite the cost and some regulatory issues that this type of envelopes pose, the prospects of long-term infection prevention and cost reduction make these envelopes an added plus to the medical device sector. Because the catheters are improving and the government supporting and encouraging the market for absorbable antibacterial envelopes market in the U. S, the future is bright promising to bring in good results for patients and cut healthcare costs.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/
- Information Technology
- Office Equipment and Supplies
- Cars and Trucks
- Persons
- Books and Authors
- Tutorials
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



